Amino acids – these are derivatives of organic acids containing one or more amino groups (NH 2) in the radical.
According to the rational nomenclature, their names have the prefix "amino" and the letters of the Greek alphabet (α-, β-, γ-, etc.), indicating the position of the amino group in relation to the carboxyl group.
4-aminobutane
(γ-aminobutyric)
Proteins contain α-amino acids, which are given trivial names.
Amino acids are subdivided into monoaminocarboxylic, diaminocarboxylic, aminodicarboxylic, aromatic and heterocyclic: CH 3 –CHNH 2 –COOH alanine (α-aminopropionic acid); H 2 N– (CH 2) 4 –CHNH 2 –COOH lysine (α, ε-diaminocaproic acid) ); COOH – CH 2 –CHNH 2 –COOH aspartic acid (α-amino succinic acid); С 6 Н 5 –СН 2 –CHNH 2 –СООН phenylalanine (α-amino-β-phenylpropionic acid);

(pyrrolidine-α-carboxylic acid).
All α-amino acids, often found in living organisms, have trivial names that are usually used in the literature.
Properties of amino acids.Amino acids are crystalline substances, usually of a sweetish taste, readily soluble in water.
Depending on the number of amino or carboxyl functional groups in an amino acid, it can have basic (lysine) or acidic properties (aspartic acid).
The amino group and the carboxyl group are capable of forming a bipolar ion or internal salt:
NH 2 –CH 2 –COOH → NH 3 + –CH 2 –СОO -.
The presence of –NН 2 and –СООН groups in the composition of amino acids determines their amphoteric properties (they interact with both bases and acids).
H 2 N – СН 2 –СООН + NaOH → H 2 N – СН 2 –COONa + Н 2 О
sodium salt of glycine
H 2 N – CH 2 –СООН + HCl → Cl -
glycine hydrochloride
Amino acids form esters with alcohols.
H 2 N – CH 2 –COOH + HOCH 3  NH 2 –СН 2 –СООСН 3 + Н 2 О
NH 2 –СН 2 –СООСН 3 + Н 2 О
glycine methyl ester
The formation of intracomplex copper salts is characteristic; the reaction can be used to isolate amino acids.

glycine copper salt
When heated, α-amino acids are converted to 1,4-diketopiperazines.

β  -Amino acids when heated easily lose ammonia, forming unsaturated acids.
-Amino acids when heated easily lose ammonia, forming unsaturated acids.
acrylic acid
γ, δ, ε-Amino acids when heated are converted into cyclic amides - lactams.

ε-aminocaproic acid caprolactam
The bulk of this product is used to obtain polyamide fiber - nylon.
Amino acids containing an amino group in the α-position undergo a dehydration reaction with the formation of polypeptides.
glycine alanine cysteine
The name of the polypeptide is made up of the names of the radicals of the acids and the full name of the acid in which the carboxyl group is retained.
Methods of obtaining.Amino acids can be obtained by hydrolysis of proteins or from halogenated acids by the action of ammonia:
α-bromopropionic acid alanine
α-Amino acids are part of the protein component of food and are divided into nonessential and irreplaceable.
Essential amino acids can be synthesized by the human or animal body. Essential amino acids (methionine, valine, leucine, phenylalanine, isoleucine, tryptophan, lysine, threonine) are not formed in a living organism and must be supplied with food for its normal functioning. For example, a lack of lysine affects hematopoietic function and bone calcification; methionine has an anti-sclerotic effect; tryptophan is important for the growth process, metabolism.
Protein substances (during hydrolysis) are a natural source for obtaining α-amino acids.
Amino acids are found in plants, microorganisms, animals and humans and play a huge role in the vital processes of organisms.
Protein – these are natural high-molecular nitrogen-containing organic compounds, the molecules of which are built from α-amino acids linked by peptide bonds, that is, proteins are polypeptides.
remainder remainder remainder
alanine serine cysteine
Proteins are widespread in nature, especially in animals and humans. A large amount of proteins is found in bones, cartilage, muscles, and nerve tissues. Hair, wool, feathers, fish scales, hooves, horns, etc. are made of proteins. Proteins are contained in the eggs of birds, are part of milk, blood, etc. Enzymes, hormones, viruses also have a protein nature.
Protein structure.All proteins contain carbon, hydrogen, oxygen, nitrogen (less often S, P, Fe).
The molecular weight of proteins is extremely high - from 5000 to many millions.
The sequence with which the residues of α-amino acids in the chain are joined is called primary structuresquirrel. It is strictly unique to each type of protein.
The polypeptide chain is usually in the form of a spiral. The tendency of the polypeptide chain to twist into a helix is \u200b\u200bpredetermined by the primary structure of the protein, but its strength is determined by the formation of hydrogen bonds between groups  and –NH– of adjacent spiral turns.
and –NH– of adjacent spiral turns.
For instance:

The peculiarity of the twisting of the polypeptide chains of protein molecules into a spiral is called secondary structureproteins.
The coils of the helix can, in one way or another, fold or bend due to the presence of disulfide bridges –S – S–, which hold certain turns in close proximity, and hydrogen bonds can also play this role. As a result of this folding of the sections of the spiral, a semblance of a ball is created. This form of protein structure is called tertiary structure.
In the molecules of some proteins, several such "tangles" (globules) are formed, as a result of which a quaternary structure appears.The bonds that connect the protein subunits into a molecule with a quaternary structure are not covalent, but weaker bonds, for example, hydrogen (as in a hemoglobin molecule), ionic or so-called hydrophobic interactions. Proteins with a quaternary structure, due to the fragility of the bonds connecting the subunits, have the ability to reversibly dissociate into subunits. In this case, the enzymatic activity of proteins may disappear.
Protein classification.Proteins are divided into two main groups: proteins (simple proteins) and proteids (complex proteins). Proteins are composed only of amino acids and do not form other products when hydrolyzed. Proteids are composed of a protein part, built from α-amino acids, and a non-protein part (prosthetic group). During hydrolysis, these proteins, in addition to α-amino acids, supply other substances: carbohydrates, phosphoric acid, heterocyclic compounds, dyes, etc.
Protein includes a number of simple proteins with different solubility in different solvents.
Albumin -proteins, soluble in water, do not precipitate with a saturated solution of sodium chloride, but precipitate with a saturated solution of ammonium sulfate, coagulate upon heating. Representatives: albumin of eggs, milk, blood serum, proteins of enzymes and plant seeds.
Globulins -proteins insoluble in water but soluble in dilute saline solutions; curdled when heated. They have a higher molecular weight than albumin. Representatives: globulins of milk, eggs, blood, muscle protein (myosin), plant seeds.
Prolamins -proteins soluble in 70–80% alcohol, insoluble in water and anhydrous alcohol. Do not curdle when boiled. Representatives: cereal proteins (gliadin - in wheat, zein - in corn).
Glutelins -vegetable proteins insoluble in neutral salt solutions and in ethyl alcohol; dissolve only in dilute (0.2%) alkali solutions. Contained mainly in cereal seeds. The glutelins of some cereals are called glutenins (from the word gluten).
Histones -proteins of a basic nature, since they contain a significant amount of diamino acids, soluble in water and in dilute acids, but insoluble in dilute alkalis. Usually protein portions of complex proteins are represented. Representatives: globin is a protein that is part of hemoglobin.
Protamines -the simplest of natural low molecular weight proteins; consist predominantly of diamino acids and are strong bases. Well soluble in water, dilute acids and alkalis. Does not coagulate when heated. Representatives of protamines are found in fish spermatozoa, in the composition of complex proteins - nucleoproteins.
Albuminoids(scleroproteins) - proteins that differ sharply from other proteins in properties. They dissolve only after prolonged treatment with concentrated acids and alkalis with the splitting of molecules. In animal organisms, they perform supporting and integumentary functions; they are not found in plants. Representatives of scleroproteins are: fibroin - silk protein; keratin - protein of hair, wool, horny substance, skin epidermis; elastin - a protein of the walls of blood vessels, tendons; collagen is a protein substance of the skin, bones, cartilage, connective tissues.
Proteids – complex proteins, subdivided into subgroups depending on the nature of the prosthetic (non-protein) part.
Phosphoproteins -proteins containing a phosphoric acid residue as a prosthetic group. Representatives: casein - milk protein, vitellin - a protein that is part of the egg yolk.
Nucleoproteins -proteins in which the actual protein part is associated with nucleic acids, and the latter upon hydrolysis form phosphoric acid, heterocyclic compounds, and carbohydrate. Nucleoproteins are part of the nuclei of plant and animal cells.
Chromoproteins -substances in which the protein part is combined with a coloring matter. Representative: blood hemoglobin, during hydrolysis it breaks down, forming a globin protein and a dye - heme.
Glucoproteins -proteins in which the protein part is combined with a carbohydrate. Representative: mucin, which is part of saliva.
Lipoproteins -compounds of proteins with lipids; the latter include fats, phosphatides, etc. Lipoproteins are found in the protoplasm of cells, in blood serum, and in egg yolk.
Protein substances are also classified according to the shape of their molecules, dividing into two groups:
a) fibrillar (fibrous) proteins, the molecules of which are filamentous; these include silk fibroin, wool keratin, muscle myosin;
b) globular proteins, the molecules of which are round; these include albumins, globulins, and a number of complex proteins.
The nutritional value of proteins is determined by the qualitative and quantitative composition of their constituent amino acids. Distinguish between complete and defective proteins.
Complete proteins contain all essential amino acids (milk casein, egg albumin, meat protein).
Defective proteins are those that do not contain at least one of the essential amino acids (corn zein, gelatin).
Protein properties.Protein substances are diverse in their state of aggregation. Often these are solid amorphous substances in the form of white powders. Wool (keratin) and silk proteins (fibroin) are strong fibers. Some proteins are obtained in a crystalline state (blood hemoglobin), many have the consistency of viscous liquids or jellies.
All proteins are insoluble in anhydrous alcohol and other organic solvents. Many proteins dissolve in water and in dilute salt solutions, but they form colloidal solutions rather than true ones. There are proteins that are completely insoluble in water.
Natural protein solutions are optically active (mainly left-handed).
Proteins, like amino acids, are amphoteric and form salts with both acids and bases:
H 3 N + –R – COO - + H + + Cl -  NH 3 + –R – COOH + Сl -;
NH 3 + –R – COOH + Сl -;
H 3 N + –R – COO - + Na + + OH -  NH 2 –R – COO - + Na + + H 2 O.
NH 2 –R – COO - + Na + + H 2 O.
In the presence of acids, protein molecules carry a positive charge and, during electrolysis, move to the cathode; in the presence of alkalis, protein molecules carry a negative charge and move to the anode. At a certain pH value, the solution will contain a minimum amount of protein cations and anions; under these conditions, the movement of protein molecules during electrophoresis does not occur. The pH value of the solution at which the protein solution contains the minimum amount of cations and anions is called isoelectric point.For different proteins, the value of the isoelectric point is not the same. For example, for egg white (albumin) the isoelectric point is at pH \u003d 4.8, for wheat protein (gliadin) - at pH \u003d 9.8.
All proteins have some common properties: precipitation reactions from solutions and color reactions.
Precipitation of proteins from solutions.When added to aqueous solutions proteins of concentrated solutions of mineral salts (for example, (NH 4) 2 SO 4) proteins are precipitated, alcohol and acetone have a similar effect on protein. In all these cases, the proteins do not change their properties and, when diluted with water, again pass into solution. This process is called salting out.
When heated, many proteins coagulate (for example, egg white) and also precipitate from solutions, but lose their ability to dissolve in water. In this case, there is a significant change in the properties of proteins. This process of irreversible precipitation of proteins is called denaturation.Denaturation can be caused by salts of heavy metals (CuSO 4, Pb (CH 3 COO) 2, HgCl, etc.), concentrated acids (HNO 3, H 2 SO 4, CH 3 COOH, picric, chloroacetic), caustic alkalis (KOH, NaOH) , phenol, formalin, tannin, etc.
Protein denaturation is also caused by various types of radiation (ultraviolet, radioactive, etc.). Denaturation destroys the quaternary, tertiary, secondary structures; In this case, the primary structure is usually preserved, that is, the destruction of peptide bonds does not occur. The primary structure of the protein is destroyed during protein hydrolysis, which occurs in the process of assimilation of protein foods. In the gastrointestinal tract, under the action of enzymes, food proteins are broken down through a number of intermediate products to amino acids. From the combination of amino acids obtained in the body, the proteins it needs are created. Unused protein residues are broken down to simpler compounds and excreted from the body.
Protein color reactions.
1. Biuret reaction.
When interacting with copper salts (CuSO 4) in an alkaline medium, all proteins give a violet color. This reaction is qualitative for peptide bonds and confirms their presence in proteins and polypeptides. For different polypeptides, the color that occurs when interacting with copper salts is not the same: dipeptides give a blue color, tripeptides — violet, and more complex polypeptides — red.
2. Xanthoprotein reaction.
When heated solutions of proteins with concentrated nitric acid (HNO 3), they turn yellow. The reaction is explained by the presence in proteins of amino acids containing aromatic hydrocarbon radicals (C 6 H 5 -), which, when interacting with nitric acid, form nitro compounds, colored yellow.
3. Cystine reaction (sulfhydryl).
If you boil a protein solution with an excess of sodium hydroxide and add a few drops of a solution of lead acetate Pb (CH 3 COO) 2, then a brown-black color or precipitate appears.
Protein + NaOH  Na 2 S + other substances.
Na 2 S + other substances.
Na 2 S + Pb (CH 3 COO) 2 → PbS ↓ + 2CH 3 COONa.
The reaction confirms the presence of weakly bound sulfur in proteins.
There are a number of other qualitative reactions to protein.
The value and application of proteins.Proteins are the most important part of food. More than 50% of proteins in the human diet should be proteins of animal origin. An adult needs 80-100 g of protein per day, and with great physical activity - 120 g or more.
Protein substances are widely used in the production of industrial goods (leather goods, wool, fur, silk), in the production of glue, gelatin, plastics (halalite), artificial wool, for the preparation of light-sensitive emulsions in the photographic industry, etc.
Many medications have a protein nature (insulin, pancreotin, etc.). Special mention should be made of biological catalysts - enzymes, which are also protein substances. Often present in only trace amounts, they promote rapid chemical transformations of huge amounts of various substances under very mild conditions (low temperatures, etc.).
Viruses, bacteria also have a protein nature. Therefore, when preserving food, treating various diseases, drugs are used that denature protein substances (salicylic acid, aspirin, salol, tannin, various polyphenols, acetic acid, etc.).
Proteins are substances that are absolutely necessary for the life of animals, plants, microorganisms. Life itself is a process of complex transformations of protein substances.
Which performs important biological functions in living organisms, participates in protein biosynthesis, is responsible for the normal activity of the nervous system and regulates metabolic processes. Artificially derived aminoacetic acid is used in pharmaceuticals, medicine and food Industry.
Food additive E640 combines aminoacetic acid (glycine) and its sodium salt under one marking number - compounds that are used to optimize the taste and aroma of products. The additive is safe and officially approved in most countries in the world.
Glycine and its sodium salt: general information
Glycine, also known as aminoacetic acid or aminoethanoic acid, belongs to the series nonessential amino acids - the simplest organic structures that are part of proteins and their compounds. The artificially obtained substance is a colorless, odorless powder and has a sweetish taste.
Glycine is produced commercially by combining chloroacetic acid and ammonia. Aminoacetic acid, in turn, has the property of forming complex salts (glycinates) with metal ions.
Sodium glycinate is a salt of sodium and aminoacetic acid, which is also a substance of synthetic origin. Despite the fact that glycine and its salt are different chemical compounds, in the food industry they perform identical functions of taste and aroma modifiers, are combined under one marking number and are considered as an E640 additive.
| Name | Glycine |
|---|---|
| Synonyms | Aminoacetic (aminoethanic) acid, glycocol (obsolete) |
| Group | Essential amino acids |
| Chemical formula | NH 2 - CH 2 - COOH |
| Structure | Small monoclinic crystals (crystal powder) |
| Color | White (colorless) |
| Smell | Absent |
| Taste | Sweet |
| Solubility | Let's completely dissolve in, partially - in. Does not dissolve in the air |
| Additive code | E640 (including sodium salt) |
| Origin | Synthetic |
| Toxicity | Safe when consumed within normal limits |
| Areas of use | Food industry, pharmaceuticals, medicine, cosmetology |
The biological role of glycine and its sources
Glycine is found in the composition of protein molecules much more often than other amino acids and performs the most important biological functions. In the human body, this amino acid is synthesized by transamination (reversible transfer of the amino group) of glyoxylate or enzymatic cleavage of choline and serine.
Aminoacetic acid is a precursor of porphyrins and purines, the biosynthesis of which occurs in living cells, but the biological role of this compound is not limited to these functions. Glycine is also a neurotransmitter that is involved in the transmission of nerve impulses, regulates the production of other amino acids and has an "inhibitory" effect on neurons and motor neurons.
The body of a healthy person independently synthesizes amino acids in the required quantities, therefore, the need for their use in the composition of medicines and dietary supplements, as a rule, is absent. Food sources of aminoacetic acid are animal products (beef liver and), nuts, and some fruits.
The effect of glycine and its sodium salt on the human body
Aminoacetic acid as a neurotransmitter performs regulatory functions and affects primarily the central and peripheral nervous system. Glycine has nootropic properties, normalizes metabolism, activates the protective functions of the central nervous system and has a mild sedative effect.
The positive effect of glycine on the human body:
- decrease in emotional stress, anxiety, stress, aggressiveness;
- improvement of mood and normalization of sleep;
- relaxing muscles and relieving cramps;
- increased efficiency;
- weakening the side effects of taking psychotropic drugs;
- decrease in the severity of vegetative-vascular disorders;
- reduced cravings for alcohol and sweets.
As part of the E640 additive, glycine and its salt do not have the above properties and do not have either a positive or negative effect on the human body when consumed within normal limits. The food supplement does not pose a threat to health, but in case of individual intolerance, it can provoke an allergic reaction.
Impurities in additives and low-quality food products, in the manufacture of which taste and aroma optimizers, are used, can pose a potential hazard.
The use of glycine and its sodium salt
The fields of application of glycine and sodium glycinate are mainly limited to the food industry, medicine and pharmaceuticals. Nevertheless, aminoacetic acid has found application in the cosmetic industry due to its hypoallergenic and antioxidant properties.
Cosmetics containing E640 additive:
- medicated shampoos for weakened hair and anti-baldness products;
- anti-aging cosmetics, moisturizers and masks for all skin types;
- cleansing serums and tonics;
- lipsticks and balms.
Crushed glycine tablets can be used in homemade skin care products and added to moisturizing masks and creams. Aminoacetic acid promotes the penetration of valuable nutrients into the deep layers of the dermis and enhances the effect of medical cosmetics.
E640 additive in the food industry
 Glycine and sodium glycinate are actively used in technological processes for the manufacture of alcoholic beverages. The additive E640, in particular, is part of the elite vodka, which allows you to neutralize the unpleasant odor and soften the harsh taste. It is also believed that the presence of glycine in alcoholic beverages helps reduce the toxic effects of alcohol on the nervous system and prevents hangovers.
Glycine and sodium glycinate are actively used in technological processes for the manufacture of alcoholic beverages. The additive E640, in particular, is part of the elite vodka, which allows you to neutralize the unpleasant odor and soften the harsh taste. It is also believed that the presence of glycine in alcoholic beverages helps reduce the toxic effects of alcohol on the nervous system and prevents hangovers.
Food products in which you can find the E640 additive:
- strong alcoholic drinks;
- jams, preserves, jellies,;
- packaged juices with pulp;
- enriched cookery;
- sports drinks fortified;
- sauces, condiments and spices.
Aminoacetic acid is used not only to optimize taste and transport biologically active substances, but also as an antibacterial agent. In particular, it is used to process meat, fish and seafood to neutralize dangerous E. coli.
Medical use
Glycine is actively used for the treatment and prevention of diseases associated with central and peripheral nervous system... This substance is a part of pharmaceutical preparations of nootropic, sedative, anticonvulsant and hypnotic effects, has a mild antidepressant and tranquilizing effect.
Medical indications for the use of aminoacetic acid as a medicine:
- decreased mental performance, sleep and memory disorders;
- emotional stress, stressful situations, neuroses;
- emotional instability and increased excitability;
- consequences of ischemic stroke, craniocerebral trauma and neuroinfections;
- vegetative-vascular dystonia, ischemia;
- increased muscle tone, muscle cramps;
- alcohol and drug addiction, toxic effects of drugs that depress the central nervous system.
It has been proven that the use of 3 g of glycine per day has a positive effect on mental abilities and the general emotional state of a person, relieves daytime sleepiness and normalizes night sleep. The drug is also prescribed for pregnant women to reduce anxiety, children and adolescents who have difficulties with social adaptation and concentration.
E640 additive and legislation
The E640 Taste and Odor Optimizer is used in food production in most countries of the world, but there is no information on the additive to Codex Alimentarius. There were no cases of poisoning with glycine and sodium glycinate when consumed with food, therefore the E640 modifier is considered safe.
The additive is included in the list of officially approved for use in the food industry in the European Union, USA and Canada. Legislation Russian Federation and Belarus also allows the presence of E640 in products within the acceptable limits established by SanPiN. There is no data on the use of E640 as a flavor enhancer and flavoring agent on the territory of Ukraine.
Despite the fact that glycine and its salt do not have a toxic effect on the human body and are approved for use, products containing E640 can hardly be called useful. Most flavors and flavors are used to draw the consumer's attention to low quality products that should be kept to a minimum.
Amino acids are organic amphoteric compounds. They contain two opposite functional groups in the molecule: an amino group with basic properties and a carboxyl group with acidic properties. Amino acids react with both acids and bases:
H 2 N -CH 2 -COOH + HCl → Cl [H 3 N-CH 2 -COOH],
H 2 N -CH 2 -COOH + NaOH → H 2 N-CH 2 -COONa + H 2 O.
When amino acids are dissolved in water, the carboxyl group eliminates a hydrogen ion, which can attach to the amino group. In this case, an internal salt is formed, the molecule of which is a bipolar ion:
H 2 N-CH 2 -COOH + H 3 N -CH 2 -COO -.
Acid-base transformations of amino acids in various media can be depicted by the following general scheme:

Aqueous solutions of amino acids have a neutral, alkaline or acidic environment, depending on the number of functional groups. So, glutamic acid forms an acidic solution (two groups —COOH, one —NH 2), lysine — alkaline (one —COOH, two —NH 2).
Like primary amines, amino acids react with nitrous acid, whereby the amino group is converted into a hydroxo group, and the amino acid into a hydroxy acid:
H 2 N-CH (R) -COOH + HNO 2 → HO-CH (R) -COOH + N 2 + H 2 O
Measuring the volume of nitrogen released allows you to determine the amount of amino acid ( van Slike method).
Amino acids can react with alcohols in the presence of gaseous hydrogen chloride, converting into an ester (more precisely, into a hydrochloric ester salt):
H 2 N-CH (R) -COOH + R'OH H 2 N-CH (R) -COOR '+ H 2 O.
Esters of amino acids do not have a bipolar structure and are volatile compounds.
The most important property of amino acids is their ability to condense to form peptides.
Qualitative reactions.
1) All amino acids are oxidized by ninhydrin

with the formation of products colored in blue-violet. The imino acid proline gives a yellow color with ninhydrin. This reaction can be used to quantify amino acids by spectrophotometry.
2) When aromatic amino acids are heated with concentrated nitric acid, the benzene ring is nitrated and compounds colored in yellow... This reaction is called xanthoprotein (from the Greek xanthos - yellow).
Glycine (Aminoacetic Acid, Glycocol, Gly, G)
H 2 NCH 2 COOH
Molecular mass 75.07; colorless crystals; melting t 232-236 ° C (with decomposition); well soluble in water, insoluble in most organic solvents. At 25 ° С p To a 2.34 (COOH) and 9.6 (NH 2); R I 5,97.
By chemical properties glycine is a typical aliphatic α-amino acid. Quantification is based on the formation of colored products with o-phthalic aldehyde (Zimmermann reaction). Found in proteins more often than other amino acids. Serves as a precursor in the biosynthesis of porphyrin compounds and purine bases. Glycine is a non-coded amino acid; its biosynthesis is carried out by transamination of glyoxylic acid, enzymatic cleavage of serine and threonine. Lycine is synthesized from chloroacetic acid and NH 3. In the D 2 O NMR spectrum, the chemical shift of the CH 2 group protons is 3.55 ppm. Internal glycine salt (CH 3) 3 + NCH 2 COO - called betaine.
Glycine is used for the synthesis of peptides, as a component of buffer solutions, in a mixture with other amino acids - for parenteral nutrition.
Chromatograms of samples containing this substance
Proteins are polypeptides of a sufficiently large molecular weight with a certain spatial structure, which depends on the sequence of amino acids included in the protein chain. This structure can be compact (globular proteins) or elongated (fibrillar proteins). All enzymes are globular proteins, while collagen and keratin are proteins of the skin and hair, which are very strong and well extensible.
According to their composition, proteins are divided into simple ones, consisting only of amino acids, and complex ones - complexes or covalent compounds polypeptides with molecules of other classes:
nucleic acids - nucleoproteins;
polysaccharides - glycoproteins;
lipids - lipoproteins;
pigments - chromoproteins;
phosphoric acid residues - phosphoproteins.
The protein part of a complex protein is called apoprotein, non-protein - prosthetic group... Almost all simple and complex globular proteins are enzymes - biological catalysts for biochemical reactions in living organisms.
Tasks
Objective 1.What volume of gas (under normal conditions) will be released during the reaction with nitrous acid 0.001 mole of the amino acid: a) leucine; b) lysine; c) proline?
Decision
Let us write down the equations for the reactions of the listed amino acids with nitrous acid. In the case of leucine and lysine, deamination occurs according to Van Slike, and in lysine - according to two amino groups contained in the molecule. There is no primary amino group in proline, so the process stops at the N-nitrosation stage.
In reaction (a), the number of moles of leucine n (Leu) is equal to the number of moles of released nitrogen n (N 2), therefore, 0.001 mol, or 22.4 ml of nitrogen, is released. In reaction (b) a doubled amount of gas 2n (N 2) is formed compared to n (lys), since there are two primary amino groups in the original amino acid; the volume of released nitrogen will be 44.8 ml. In reaction (c), nitrogen is not released: proline is the only one of the 20 most important amino acids, which has a secondary nitrogen atom in the a-position. When nitrosated, an N-nitroso (not diazo) compound is formed.
Answer:a) 22.4 ml of N 2; b) 44.8 ml of N 2; c) there is no release of N 2.
Objective 2.As a result of the treatment of a solution containing 9.36 mg of an unknown amino acid with an excess of nitrous acid at 748 mm Hg. and 20 ° C., 2.01 ml of nitrogen was obtained. What is the minimum molar mass this amino acid?
Decision
Reaction of amino acid with nitrous acid:

Using the combined gas law PV / T \u003d const, we calculate the volume of nitrogen under normal conditions (at a temperature of 0 ° C, or 273 ° K, and a pressure of 760 mm Hg): V 0 (N 2) (n.o.) \u003d (P 1 V 1 T 0) / (P 0 T 1) \u003d (748 2.01 273) / (760 293) \u003d 1.84 ml.
Since the quantities of moles of the amino acid substance and the released gas N2 are the same, we will compose the proportion:
9.36 mg of the amino acid gives 1.84 ml of N 2;
M (molecular weight of the amino acid) will give \u003d 22.4 N 2.
M \u003d (9.36 22.4) / 1.84 \u003d 114.
It could be valine: 
Objective 3.Animal hemoglobin contains 0.335% iron (by weight). What is the minimum molar mass of this protein? How many iron atoms are in a molecule if the molecular weight of the protein determined by measuring the osmotic pressure is 67,000?
Decision
Mass fraction of iron in hemoglobin: С% (Fe) \u003d / M (protein), where n is the number of Fe atoms in a protein molecule. Let n \u003d 1, then M (protein) 1 \u003d M (Fe) · 100% / C% (Fe) \u003d 56 · 100 / 0.335 \u003d 16700. Since the true value (by condition) of M (protein) ist. \u003d 67000, n \u003d M source / M 1 \u003d 67000/16700 \u003d 4.
Task 4.During alkaline hydrolysis of 48 g of a dipeptide consisting of residues of the same amino acid, only one substance was formed - the sodium salt of the amino acid. The mass of this salt is 66.6 g. Establish the structure of the dipeptide.
Decision
The equation for the reaction of alkaline hydrolysis of a dipeptide:
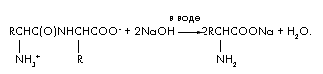
Let's designate the amount of dipeptide in moles (dipeptide) \u003d x. Then, in the hydrolysis reaction, twice the amount of alkali (NaOH) \u003d 2x is consumed and (amino acids) \u003d 2x and (H2O) \u003d x are formed. Let's compose the material balance equation using the formula m \u003d · M;
m (dipeptide) + m (NaOH) \u003d m (amino acid salt) + m (H 2 O); 48 + 2x 40 \u003d 66.6 + x 18; 62x \u003d 18.6; x \u003d 0.3 mole. M (dipeptide) \u003d m / \u003d 48 / 0.3 \u003d 160. M (amino acids) \u003d [M (dipeptide) + M (H 2 O)] / 2 \u003d 178/2 \u003d 89 g / mol. The amino acid is alanine. Dipeptide structure: ![]() .
.
