Organic matter in wildlife
Organic substances are the basis of all living nature. Plants and animals, microorganisms and viruses - all living things consist of a huge amount of various organic substances and a relatively small number of inorganic ones. It was carbon compounds, due to their great diversity and ability to numerous chemical transformations, that were the basis on which life arose in all its manifestations. The carriers of the properties that are included in the concept of "life" are complex organic substances, the molecules of which contain chains of many thousands of atoms - biopolymers.
First of all it is proteins -carriers of life, the basis of a living cell. Complex organic polymers - Proteins are composed primarily of carbon, hydrogen, oxygen, nitrogen, and sulfur. Their molecules are formed by combining a very large number of simple molecules - the so-called amino acids(see article "Chemistry of Life").
There are many different proteins. There are supporting proteins, or structural ones. Such proteins are part of the bones, form cartilage, skin, hair, horns, hooves, feathers, fish scales. Muscle composition structural proteins are included together with proteins that perform contractile functions. Muscle contraction (the most important role of this type of protein) is the conversion of part of the chemical energy of such proteins into mechanical work. A very large group of proteins regulates chemical reactions in organisms. it enzymes(biological catalysts). Currently, more than a thousand of them are known. Highly developed organisms are also able to produce protective proteins - the so-called antibodies, which are able to precipitate or bind and thereby neutralize foreign substances and bodies that have entered the body from the outside.
Along with proteins, the most important functions of life are nucleic acids.In a living organism, metabolism always takes place. The composition of almost all of its cells is constantly renewed. Cell proteins are also renewed. But for each organ, for each tissue, you need to make its own special protein, with its own unique order of amino acids in the chain. The keepers of this order are nucleic acids. Nucleic acids are a kind of templates by which organisms build their proteins. It is often figuratively said that they contain the protein synthesis code. Each protein has its own code, its own template. Nucleic acids have another function. They are templates for the nucleic acids themselves. This is a kind of "memory device" with the help of which each species of living creatures transmits from generation to generation the codes for the construction of their proteins (see the article "Chemistry of Life").
Proteins are not the only ones that perform support functions in nature. In plants, for example, supporting, skeletal substances - cellulose and lignin. These are also polymeric substances, but of a completely different type. The long chains of cellulose atoms are built from glucose molecules from the sugar group. Therefore, cellulose is referred to as polysaccharides. The structure of lignin has not yet been finally established. This is also a polymer, apparently with reticulated molecules. And in insects, chitin, also a polysaccharide, performs supporting functions.
There is a large group of substances (fats, sugars, or carbohydrates) that carry and store chemical energy. They (together with food proteins) are a spare building material necessary for the formation of new cells (see the article "Food Chemistry"). A lot of organic substances (vitamins, hormones) in living organisms play the role of life regulators. Some regulate respiration or digestion, others - the growth and division of cells, others - the activity of the nervous system, etc. Living organisms contain numerous substances for a wide variety of purposes: coloring, to which the world of flowers owes its beauty, odorous - attracting or repelling, protecting against external enemies, and many others. Plants and animals, even every single cell, are small but very complex laboratories in which thousands of organic substances arise, transform and decompose. Numerous and varied chemical reactions take place in these laboratories in a strictly defined sequence. The most complex structures are created, grow and then disintegrate ...
The world of organic substances surrounds us, we ourselves consist of them, and all living nature, among which we live and which we constantly use, consists of organic substances.
The structure of a natural polymer - silk fibroin protein. Individual polymer chains are linked by hydrogen bonds (dotted line).
Our review, in which cells are viewed as units of living matter, cannot be complete if we do not touch on viruses. Although viruses are not living, they are biologically formed supramolecular complexes that are capable of self-replication in their respective host cells. The virus consists of a nucleic acid molecule and its surrounding protective shell, or capsid, built of protein molecules. Viruses exist in two states.
Figure: 2-23. Electron micrograph of the plant cell wall. The wall consists of crisscrossing layers of cellulose fibers immersed in organic glue. The walls of plant cells are very strong, in their structure they resemble a concrete slab reinforced with steel reinforcement.
Figure: 2-24. Replication of a bacteriophage in a host cell.
Some viruses contain DNA, while others contain RNA.
Hundreds of different viruses are known that are specific for certain types of host cells. The role of hosts can be played by cells of animals, plants, or bacteria (Table 2-3). Viruses specific to bacteria are called bacteriophages, or simply phages (the word phage means to eat, to consume). The capsid of viruses can be built from protein molecules of only one type, as is the case, for example, in the case of the tobacco mosaic virus, one of the simplest viruses, which was the first to be obtained in crystalline form (Fig. 2-25). Other viruses can contain tens or hundreds of different types of proteins. The size of viruses varies widely. Thus, one of the smallest viruses, bacteriophage fKh174, has a diameter of 18 nm, while one of the largest viruses, the vaccinia virus, corresponds to the smallest bacteria in particle size. Viruses also differ in shape and complexity of their structure. Among the most complex is bacteriophage T4 (Fig. 2-25), for which E. coli serves as a host cell. Phage T4 has a head, a process (“tail”), and a complex set of tail filaments; when viral DNA is introduced into a host cell, they act together as a "sting" or hypodermic syringe. In fig. 2-25 and in table. 2-3 shows data on the size, shape and mass of particles of a number of viruses, as well as the type and size of their constituent nucleic acid molecules. Some viruses are unusually pathogenic to humans. These include, in particular, viruses that cause smallpox, polio, influenza, colds, infectious mononucleosis and shingles. It is believed that viruses, which can be latent, are also the cause of cancer in animals.
Table 2-3. Properties of some viruses

Viruses play an increasingly important role in biochemical research, since they can be used to obtain unusually valuable information about the structure of chromosomes, the mechanisms of enzymatic synthesis of nucleic acids, and the regulation of the transfer of genetic information.
Carbohydrates are composed of ...
carbon, hydrogen and oxygen
carbon, nitrogen and hydrogen
carbon, oxygen and nitrogen
Carbohydrates, or saccharides, is one of the main groups organic compounds... They are part of the cells of all living organisms. Carbohydrates are made up of carbon, hydrogen and oxygen. They got their name because most of them have the same ratio of hydrogen and oxygen in a molecule as in a water molecule.
General formula of carbohydrates: Сn (Н 2 О) m. Examples include glucose - C 6 H 12 O 6 and sucrose - C 12 H 22 O 11. Other elements can also be included in carbohydrate derivatives. All carbohydrates are divided into simple, or monosaccharides, and complex, or polysaccharides... Of the monosaccharides, ribose, deoxyribose, glucose, fructose, galactose are of the greatest importance for living organisms.
Functions of carbohydrates: energetic, building, protective, storage.
Determined from the proposed polysaccharides.
starch, glycogen, chitin ...
glucose, fructose, galactose
ribose, deoxyribose

Di- and polysaccharides are formed by combining two or more monosaccharides. Disaccharides are similar in properties to monosaccharides. Both are highly water soluble and have a sweet taste. Polysaccharides are composed of a large number of monosaccharides combined covalent bonds... These include starch, glycogen, cellulose, chitin other.
Violation of the natural structure of the protein.
denaturation
renaturation
degeneration

Violation of the natural structure of the protein is called denaturation... It can occur under the influence of temperature, chemicals, radiant energy, and other factors. With a weak impact, only the quaternary structure disintegrates, with a stronger one, the tertiary structure, and then the secondary one, and the protein remains in the form of a polypeptide chain. This process is partially reversible: if the primary structure is not destroyed, then the denatured protein is able to restore its structure. Thus, all the structural features of the protein macromolecule are determined by its primary structure.
The function that accelerates bio chemical reactions in a cage.
catalytic
enzymatic
both answers are correct

Function of contractile proteins.
motor
transport
protective

The flagellum of all eukaryotic cells is about 100 μm long. On the cross section, it can be seen that 9 pairs of microtubules are located along the periphery of the flagellum, and 2 microtubules are located in the center. All pairs of microtubules are interconnected. The protein that carries out this binding changes its conformation due to the energy released during the hydrolysis of ATP. This leads to the fact that pairs of microtubules begin to move relative to each other, the flagellum bends and the cell begins to move.
The function of proteins, due to which hemoglobin carries oxygen from the lungs to the cells of other tissues and organs.
transport
motor
both answers are correct
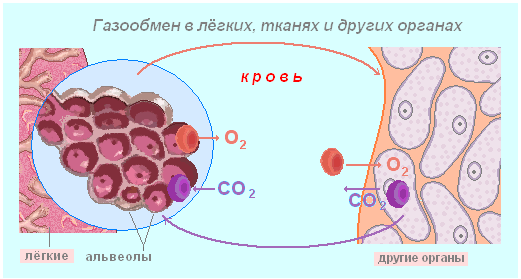
It is important transport function of proteins. So, hemoglobin carries oxygen from the lungs to the cells of other tissues and organs. In muscles, this function is performed by the protein hemoglobin. Serum proteins (albumin) promote the transfer of lipids and fatty acids, various biologically active substances... By attaching oxygen, hemoglobin from bluish color becomes scarlet. Therefore, blood, in which there is a lot of oxygen, differs in color from blood in which there is little oxygen. Transport proteins in the outer membrane of cells carry various substances from environment into the cytoplasm.
Protein function that maintains a constant concentration of substances in the blood and body cells. Participate in growth, reproduction and other vital processes.
enzymatic
regulatory
transport

The DNA molecule contains nitrogenous bases ...
adenine, guanine, cytosine, thymine
adenine, guanine, leucine, thymine
there is no right answer

The DNA molecule contains four types of nitrogenous bases: adenine, guanine, cytosine and thymine. They determine the names of the corresponding nucleotides.
Determine the composition of the nucleotide.
phosphoric acid residue, cytidine, carbohydrate
nitrogenous base, carbohydrate, DNA
nitrogenous base, carbohydrate, phosphoric acid residue

Each nucleotide consists of three components connected by strong chemical bonds... This is a nitrogenous base, a carbohydrate (ribose or deoxyribose) and a phosphoric acid residue.
The name of the bond between adenine and thymine in the formation of a double-stranded DNA molecule.
single
double
triple

A DNA molecule is a double row of nucleotides, stitched in the longitudinal and transverse directions The framework of its structure is carbohydrates reliably linked by phosphate groups in two chains. Between the chains "ladder" are nitrogenous bases attracted to each other by weak hydrogen bonds (in the case of adenine-thymine, the bond double).
Determine the composition of adenosine triphosphate:
adenine, uracil, two phosphoric acid residues
adenine, ribose, three phosphoric acid residues

Nucleic acid adenosine triphosphate (ATP) is made up of a single nucleotide and contains two high-energy (energy-rich) bonds between phosphate groups. ATP is absolutely essential in every cell, as it plays the role of a biological accumulator - an energy carrier. It is needed wherever energy is stored or released and used, that is, in almost any biochemical reaction, since such reactions occur in every cell almost continuously, each aTP molecule discharges and recharges, for example, in the human body, on average once a minute. ATP is found in the cytoplasm, mitochondria, plastids and nuclei.
virus
Assignments for part A. Choose one correct answer from the four suggested
A1. The lowest level of organization of the living is:
1) atomic
2) cellular
3) molecular
4) organismic
A2. Among the substances listed, it is not a biological polymer:
2) glucose
3) glycogen
4) hemoglobin
A3. Inorganic substances cells are:
1) carbohydrates and fats
2) nucleic acids and water
3) proteins and fats
4) water and mineral water
A4. Organic substances of a cell, which provide storage of hereditary information and transmission to its descendants, are the basis of its genetic apparatus:
3) carbohydrates
4) nucleic acids
A5. Of the listed carbohydrates, the monosaccharide is:
2) starch
3) sucrose
4) fructose
A6. Lipid molecules are composed of:
1) amino acid
2) monosaccharides
3) water and minerals
4) glycerin and higher fatty acids
A7. Compared to the oxidation of 1 g of carbon, the oxidation of fats of the same mass produces energy:
1) half as much
2) twice more
3) four times more
4) the same amount
A8. Organic substances, which are the main building material of cell structures and take part in the regulation of the processes of its vital activity, are:
1) proteins
3) carbohydrates
4) nucleic acids
A9. The whole variety of proteins is formed due to the various combinations in their molecules:
1) 4 amino acids
2) 20 amino acids
3) 28 amino acids
4) 56 amino acids
A10. The highest level of spatial structural configuration of the hemoglobin molecule:
1) primary
2) secondary
3) tertiary
4) quaternary
A11. Monomers of nucleic acid molecules are:
1) nucleotides
2) monosaccharides
3) amino acids
4) higher fatty acids
A12. The DNA contains sugar:
2) glucose
3) fructose
4) deoxyribose
A13. Indicate a pair of complementary nucleotides in a DNA molecule:
2) AT
A14. For the DNA site ACCGTAATG, indicate the complementary strand:
1) AAGGTSAGT
2) THGTSTAACTS
3) TCTSGTTATSG
4) TGGZATTATS
A15. ATP includes:
1) ribose, adenine, three phosphoric acid residues
2) ribose, adenine, one phosphoric acid residue
3) ribose, deoxyribose, three phosphoric acid residues
4) deoxyribose, adenine, three phosphoric acid residues
A16. ATP plays an important role in the metabolism of organisms because:
1) is the structural basis of nucleotides
2) contains microenergy communication
3) is usually the end product of metabolism
4) it can be quickly obtained from the environment surrounding the body
A17. Vitamins are water-soluble:
2) C
A18. By chemical composition most enzymes are:
2) proteins
3) carbohydrates
4) nucleic acids
2) viruses
3) bacteria
4) unicellular plants
A20. Viruses are composed of:
1) cellulose membrane, cytoplasm, nucleus
2) protein membrane and cytoplasm
3) nucleic acid and protein coat
4) several microscopic cells
Assignments for part B. Choose three correct answers from the six proposed
IN 1. The DNA molecule differs from mRNA in that:
1) it is coiled
2) consists of two polynucleotide chains
3) consists of one polynucleotide chain
4) has the ability to self-double
5) does not have the ability to self-double
6) serves as a matrix for the assembly of the polypeptide chain
AT 2. Carbohydrates have the following functions:
1) signal
2) structural
3) transport
4) regulatory
5) energy
6) enzymatic
Match the content of the first and second columns
AT 3. Relate organic matter and the function it performs in the cell and / or in the body
and b in r d 5 1 4 2 3
Establish the correct sequence of biological processes, phenomena, practical actions
AT 4. Set the sequence of the formation of the structure of the hemoglobin protein molecule
a) twisting of protein molecules into a spiral
b) the formation of peptide bonds between amino acids and the formation of a peptide chain
c) combining several globules
d) twisting the protein molecule into a ball
